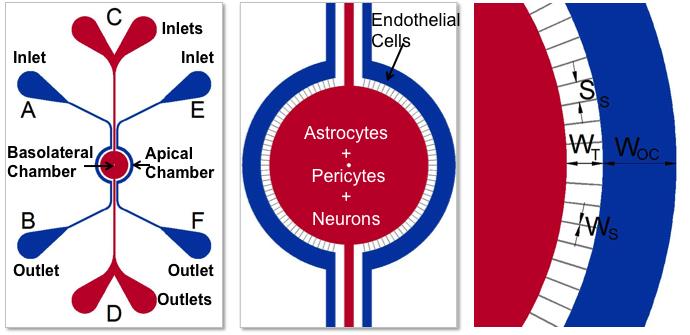
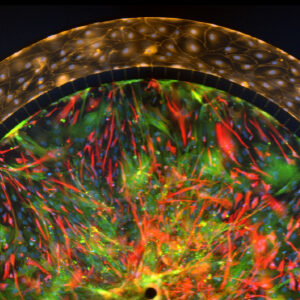
SynBBB blood-brain barrier model recreates the in vivo microenvironment by replicating a histological slice of brain tissue cells. The model is able to do this by communicating with endothelial cells across the blood-brain barrier (BBB). As a result, shear-induced endothelial cell tight junctions are easily achieved in the SynBBB model using physiological fluid flow. This is something that cannot be achieved in other models such as the Transwell® model. The formation of tight junction changes can be measured using biochemical or electrical analysis (assessing changes in electrical resistance) with the SynVivo Cell Impedance Analyzer. Interactions between brain tissue cells and endothelial cells are readily visualized in the SynBBB assay. Transwell® models do not allow real-time visualization of these cellular interactions, which are critical for understanding the BBB microenvironment.
Highlights include:
- Accurate in vivo hemodynamic shear stress
- Real-time visualization and quantitation of cellular and barrier functionality
- Significant reduction in cost and time
- Robust and easy to use protocols
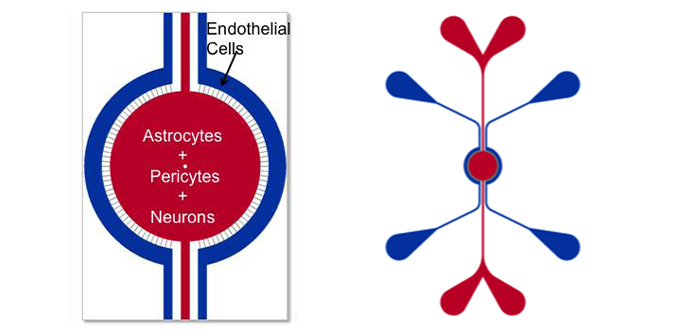
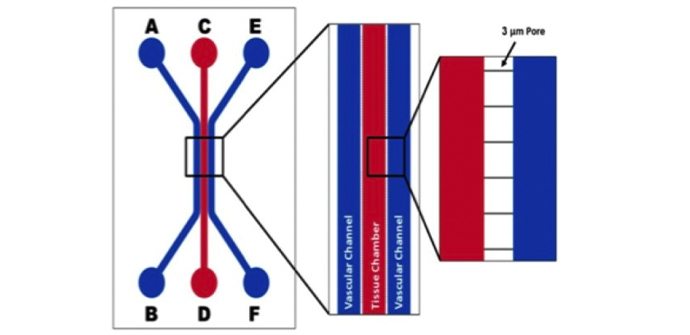
Schematics of the devices used to develop the BBB Model. Apical chamber (outer channels) are for culture of vascular (endothelial cells) while basolateral chamber (central chamber) are for culture of brain tissue cells (astrocytes, pericytes, or neurons). Porous architecture enables communication between the vascular and tissue cells. Outer Channel Width (OC), Travel Width (T), Slit Spacing (SS), Slit Width (WS).
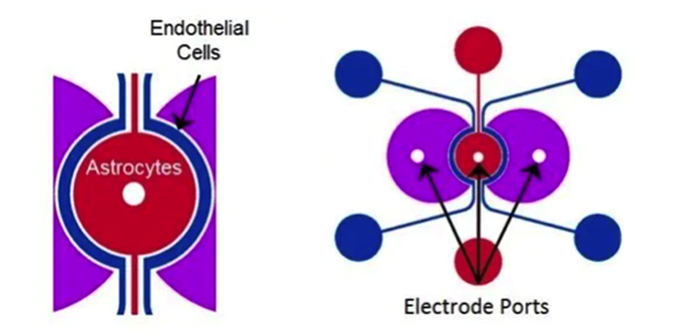
Types of Models Functionalized in the BBB Devices
PRODUCT PURCHASING OPTIONS
Chips: Depending on your specific research applications you can select from basic IMN2 (radial or linear) or IMN2 radial “TEER-compatible” chip configurations.
Kits: All the basic components required to run SynBBB assays can be purchased in a kit format. Choose between the IMN2 Radial, IMN2 Linear, or IMN2 Radial TEER Chips.
Starter Kit: Select this for your first-time purchase
- 12 SynBBB chips (Choice of IMN2 radial, IMN2 linear or IMN2 radial-TEER co-culture chips)
- Accessories including tubing, clamps, needles, and syringes
- Pneumatic priming device (required for priming tubing to remove air)
- Cell impedance analyzer* (required for SynBBB TEER measurements)
*included in the IMN2-TEER starter kit only
Idealized Co-Culture Network Chips (IMN2 radial)
IMN2 radial Chip (200μm Outer channel, 1.8mm tissue chamber, 50μm slit spacing, 3um wide slit, 50μ travel (space between channels, 100μm depth (height)
Starter Kit
$2,100Add to cart
Idealized Co-Culture Network Chips (IMN2 TEER)
IMN2 radial-TEER Chip: 200μm Outer channel, 1.8mm tissue chamber, 50μm slit spacing, 3um wide slit, 50μ travel (space between channels), 100μm depth (height), w/impedance capability
Starter Kit
$3,600Add to cart
Idealized Co-Culture Network Chips (IMN2 Linear)
IMN2 Linear Chip: 200um-500um-200um Channel Widths; 50um Travel (distance between channels)- 3um slits-50um Separation, 100um Depth (height)
Starter Kit
$2,100Add to cart
SynVivo Platform Used to Create the First Neonatal Blood-Brain Barrier on a Chip
Researchers at Temple University used the SynBBB brain on a chip to model the attributes and functions of the neonatal blood-brain barrier (BBB). The SynBBB model closely mimics the in vivo microenvironment including three-dimensional morphology, cellular interactions, and flow characteristics on a microfluidic chip. This work marked the first dynamic in vitro neonatal BBB model that offers real-time visualization and analysis suitable for studies of BBB function and screening of novel therapeutics.
A Novel Dynamic Neonatal Blood-Brain Barrier on a Chip
Authors: S. Deosarkar, B. Prabhakarpandian, B. Wang, J.B. Sheffield, B. Krynska, M. Kiani.
PLOS ONE, 2015, DOI: 10.1371/journal.pone.0142725
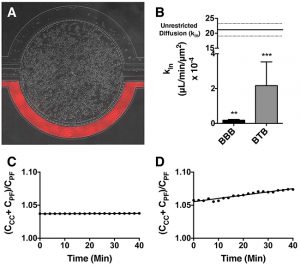
SynBBB models include a tissue compartment and vascular channels placed side-by-side and separated by an engineered porous barrier. Therefore, the researchers were able to co-culture neonatal rat brain endothelial cells and rat astrocytes under physiological conditions observed in vivo. The endothelial cells formed a full lumen and exhibited tight junction formation, which increased under co-culture with astrocytes. Small molecule permeability in the blood-brain barrier on a chip was found to be in excellent agreement with in vivo observations. SynBBB exhibits significantly improved barrier function compared to the Transwell model and closely approximates the permeability of in vivo BBB.
SynVivo Platform Used to Develop Blood Tumor Barrier On-A-Chip
Permeability Across a Novel Microfluidic Blood-Tumor-Barrier Model
Authors: Tori B. Terrell-Hall, Amanda G. Ammer , Jessica I. G. Griffith and Paul R. Lockman
Fluids and Barriers of the CNS (2017) 14:3
In this study, Dr. Lockman’s team adapted the SynVivo BBB model to develop and characterize a BTB model. Results from the study demonstrate that the in vitro BTB model mimics the in vivo BTB with regard to permeability and efflux properties. In addition, inhibitor-based modulation of the permeability was readily quantified and compared very well with in vivo observations. According to Dr. Lockman “While some in vitro models have a flow component, this assay is the first-ever blood-tumor barrier developed using a commercially available microfluidic model with shear stress similar to that observed in vivo in addition to real-time visualization and quantitation.” This Blood Tumor Barrier model lays the foundation for use in screening assays for drug discovery and understanding of Central Nervous System diseases.
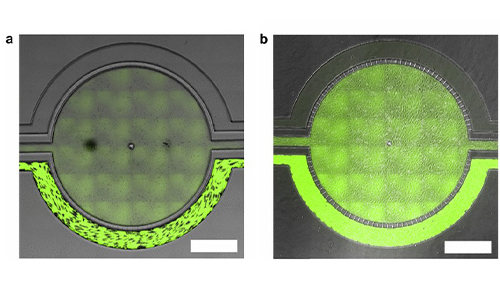
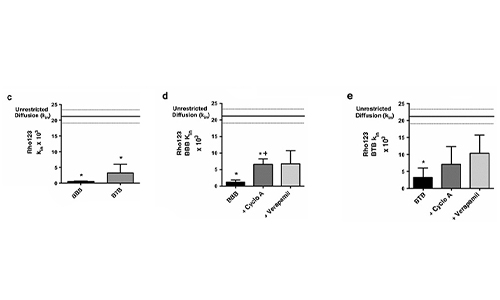
Representative brightfield image of Rhodamine 123 dye accumulation in the central compartment after 90 min of perfusion in the BBB model without an inhibitor (a) and with an inhibitor (b). Rate of fluorescent dye accumulation of Rho123 into central compartment after 90 min of dye perfusion in BBB, and BTB chips (c). Rate of fluorescent dye accumulation in BBB (d) and BTB (e) chips perfused with Rho123 ± P-gp inhibitors. Statistical significance was determined using one-way ANOVA followed by Tukey’s multiple comparison tests, and student’s t test; *p < 0.05 significance between tracer and unrestricted diffusion kin, n = 3–4; +p < 0.05 significance between BBB/BTB models and the addition of inhibitor, n = 3–6. All data represent mean ± SEM. White rectangle scale bars 500 μm
SynBBB Blood Tumor Barrier On-A-Chip Advances Understanding of Therapeutic Transfer Across the Blood-Brain Barrier
Trastuzumab Distribution in an In-Vivo and In-Vitro Model of Brain Metastases of Breast Cancer
Authors: Tori B. Terrell-Hall, Mohamed Ismail Nounou, Fatema El-Amrawy, Jessica I.G. Griffith and Paul R. Lockman
Oncotarget. 2017; 8:83734-83744
Researcher Paul Lockman and colleagues at West Virginia University, report on one of the first studies to monitor and quantify Trastuzumab movement across the blood-brain barrier using the SynVivo Blood-Brain Barrier (BBB) on-a-chip. Trastuzumab is a monoclonal antibody that is a widely used therapeutic for the treatment of HER2+ breast cancer. In the study published in Oncotarget titled “Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer”, SynVivo’s BBB model was adapted to develop a blood tumor barrier (BTB) model. The model was comprised of HER2+ breast cancer cells followed by real-time monitoring of the tissue distribution of the antibody trastuzumab. Data showed that the permeability of trastuzumab in-vivo increased from the BBB to the BTB similar to that observed in the SynVivo model.
According to Dr. Lockman, “Development of an in vitro model with the ability to predict in vivo responses across the BBB is critical to progress our understanding and therapeutic strategies for brain disorders”. SynVivo’s in vivo validated microfluidic 3D tissue models provide a valuable resource for augmenting translational research for drug discovery and delivery.
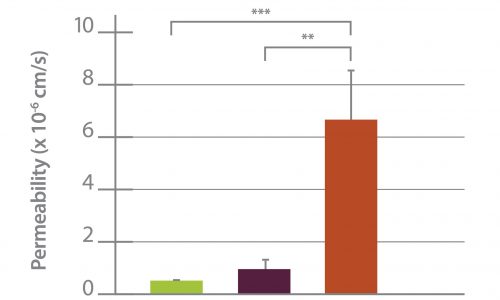
Mechanism of trastuzumab movement. Linear central compartment accumulation of t-Rho123 in in-vitro BBB and BTB microfluidic chip models. Representative image of model with TRITC labeled t-Rho123 flowing over HUVEC cells in the outer compartment and either astrocytes or JIMT-1 cancer cells in the central compartment (A). Rate of t-Rho123 movement in each model plotted against the unrestricted diffusion kin; ** p<0.0033 significance between BBB model and unrestricted diffusion kin, n=3; *** p<0.0005 significance between BTB model and unrestricted diffusion kin, n=3. All data represent mean ± S.E.M. Each model is significantly different than 0 (p < 0.05) (B). Representative graphs of the rate of accumulation of t-Rho123 in the BBB (C) and BTB
SynBBB Blood-Brain Barrier On-A-Chip Assay Used For Screening of Novel Therapeutics in Real-Time
Protein Kinase C-Delta Inhibition Protects Blood-Brain Barrier from Sepsis-Induced Vascular Damage
Authors: Yuan Tang, Fariborz Soroush, Shuang Sun, Elisabetta Liverani, Jordan C. Langston, Qingliang Yang, Laurie E. Kilpatrick, and Mohammad F. Kiani. J
Neuroinflammation. 15: 309 (2018).
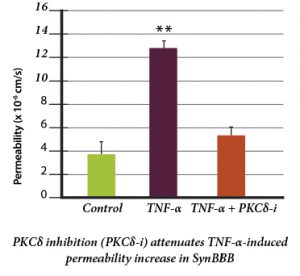
This publication reports on the use of the blood-brain barrier model to elucidate the regulation and relative contribution of Protein Kinase C-delta in the control of individual steps in neuroinflammation during sepsis. The role of PKC-delta-TAT peptide inhibitor as a potential therapeutic for the prevention or reduction of cerebrovascular injury in sepsis-induced vascular damage was also studied.
Vascular integrity was assessed using the SynBBB Co-Culture model with primary human brain microvascular endothelial cells and Astrocytes. Endothelial cell permeability, TEER, and neutrophil transmigration were directly evaluated. SynBBB also allowed for real-time monitoring of neutrophil-endothelial interaction under physiologically relevant flow conditions.
PKCδ activation is a key signaling event that alters the structural and functional integrity of BBB leading to vascular damage and inflammation-induced tissue damage. PKCδ-TAT peptide inhibitor has therapeutic potential for the prevention or reduction of cerebrovascular injury in sepsis-induced vascular damage.
Human Blood-Brain Barrier on a Chip Developed with hCMEC/D3 Brain Endothelial Cell Line and Primary Human Astrocytes
A Microfluidic Model of Human Brain (uHuB) for Assessment of Blood-Brain Barrier
Authors: Tyler D. Brown, Maksymillian Nowak, Alexandra V. Bayles, Balabhaskar Prabhakarpandian, Pankaj Karande, Joerg Lahann, Matthew Helgeson, Samir Mitragotri.
Bioengineering and Translational Medicine. 15: 309 (2019; 4:e10126)
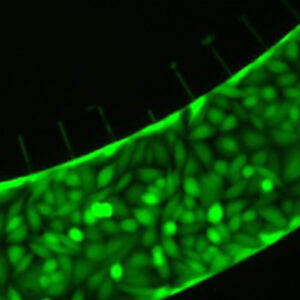
hCMEC/D3 cell line forms a complete lumen underflow and displays the appropriate tight junction markers.
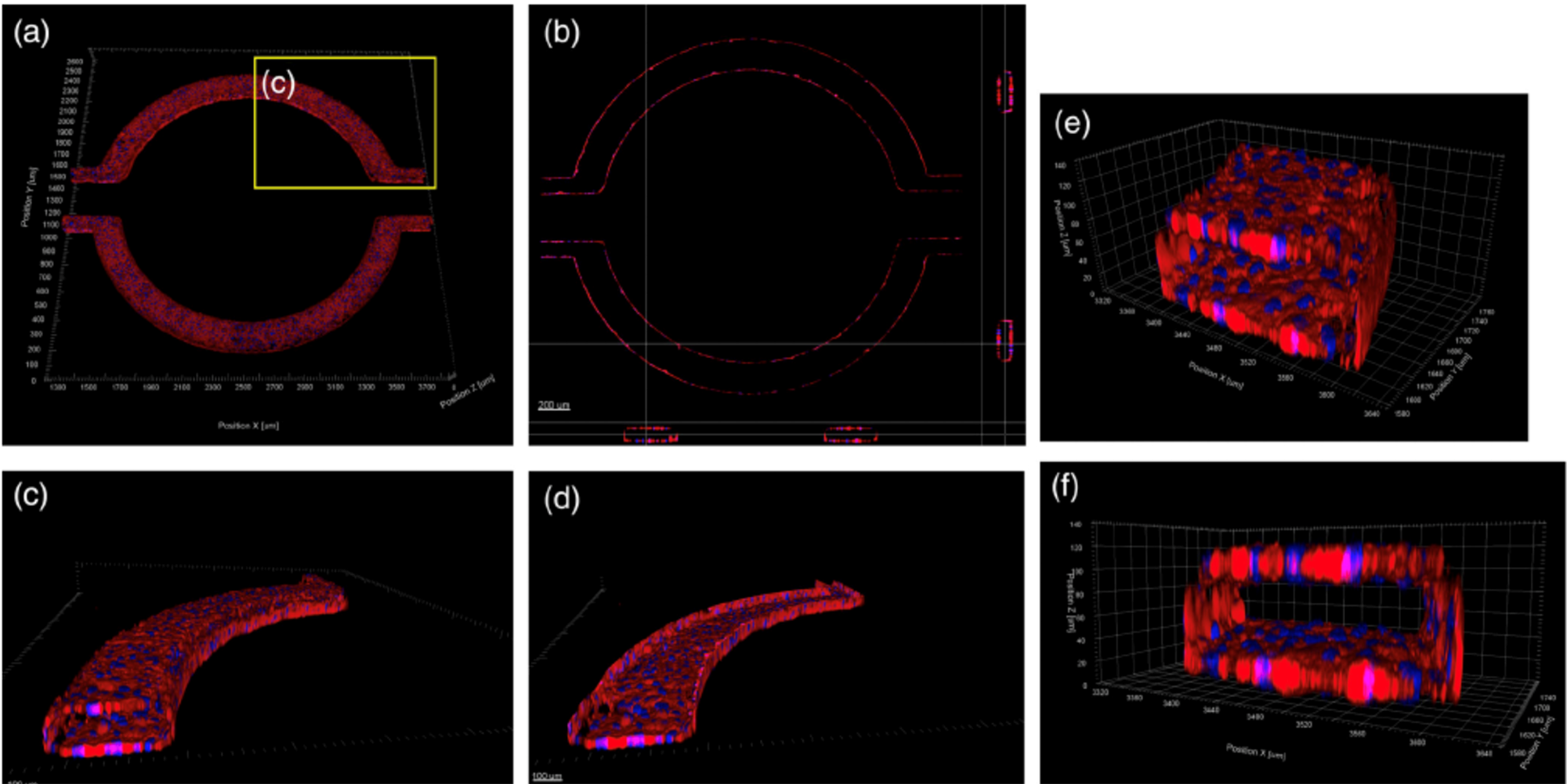
(a–f) Confocal images of hCMEC/D3 monolayers in the μHuB after conditioning to flow stained with ActinRed™ 555 ReadyProbes™ (actin, red) and Hoechst 33342 (nucleus, blue). (a) Onward‐looking view of μHuB device consisting of two vascular (apical) compartments lined with hCMEC/D3 monolayers. (b) Cross‐sectional view of hCMEC/D3 monolayers in μHuB forming a complete inner lumen approximately 200 μm (width) by 100 μm (height). (c) Onward‐looking view of one quadrant of the μHuB model as outlined in yellow in (a). (d) Lower half of (c), lined with a complete hCMEC/D3 monolayer. (e) Cross‐sectional view of inner lumen. (f) Same cross‐section as (e) at 90° viewing angle
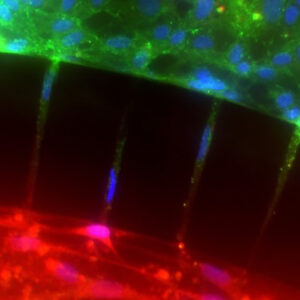
Co-Culture of hCMEC/D3 and Primary Astrocytes in the Human SynBBB Blood-Brain Barrier On-A-Chip
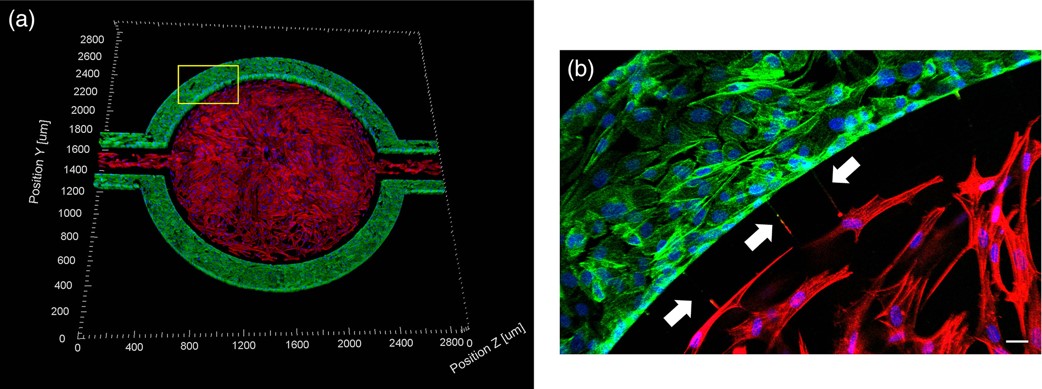
hCMEC/D3 monolayers (green) were cultured in the vascular (apical) compartments with primary human astrocytes (red) in the tissue (basolateral) compartment (nuclei, blue). (a) Onward‐looking view of complete, three‐dimensional reconstruction of the co culture SynBBB model. (b) Zoomed‐in yellow region of (a) with arrows pointing to regions where astrocyte end‐feet are protruding to hCMEC/D3 monolayer. (scale bar for b = 20 μm
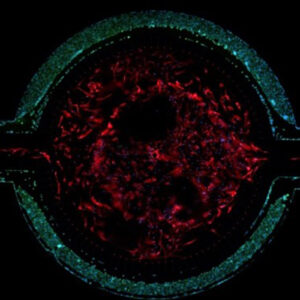
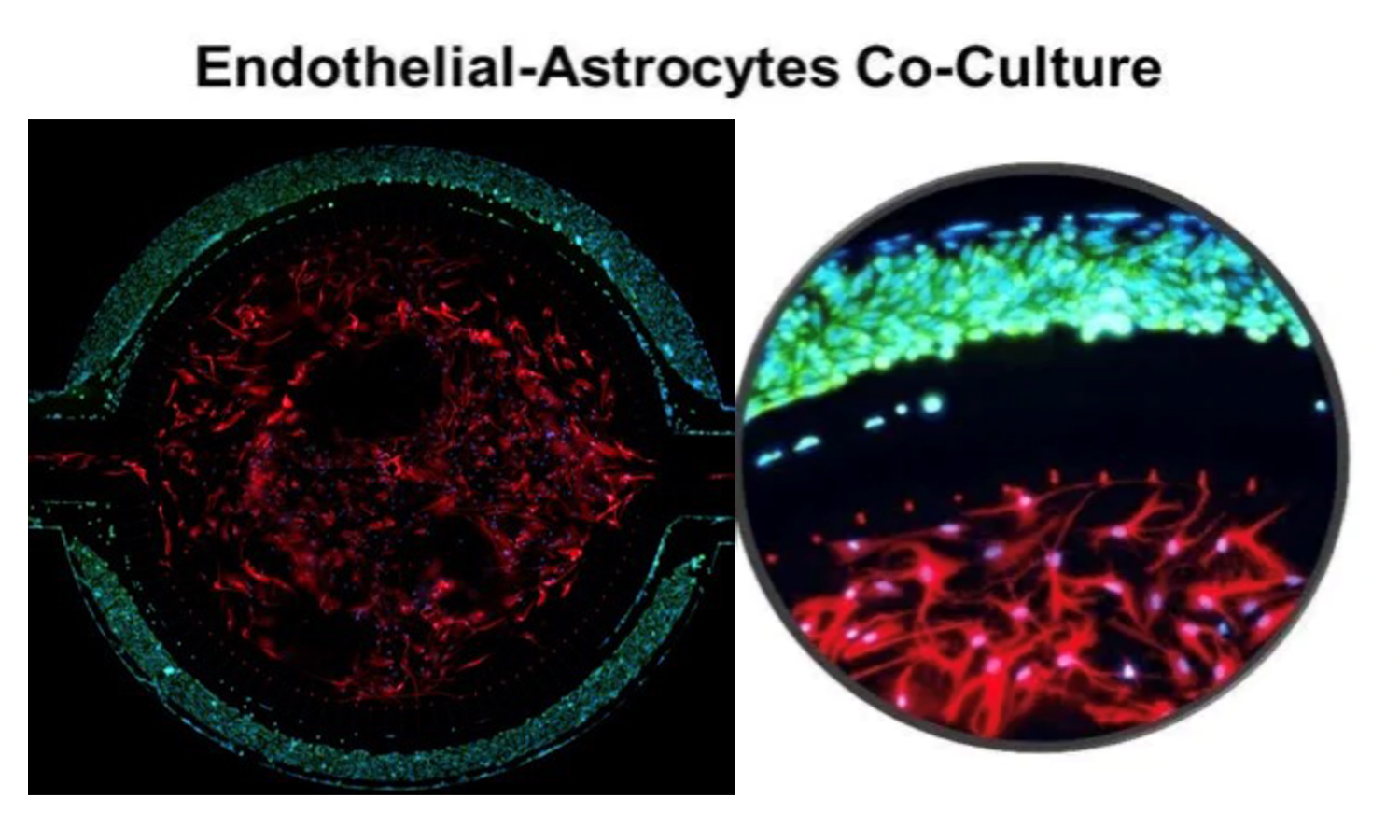
Co-Culture with Endothelial Cells and Astrocytes
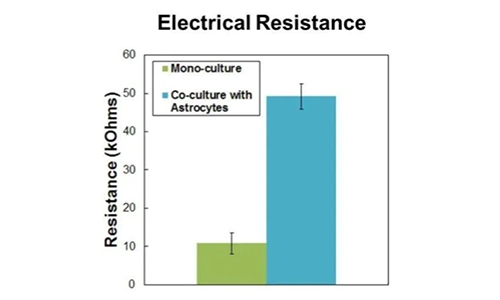
Primary rat brain endothelial cells and astrocytes co-cultured in the SynBBB device. Increases in Ohmic resistance across the barrier, measured with the Cell Impedance Analyzer, are associated with tight junction formation across the BBB. Endothelial cells co-cultured with astrocytes form significantly tighter cell junctions compared to mono-cultured endothelial cells.
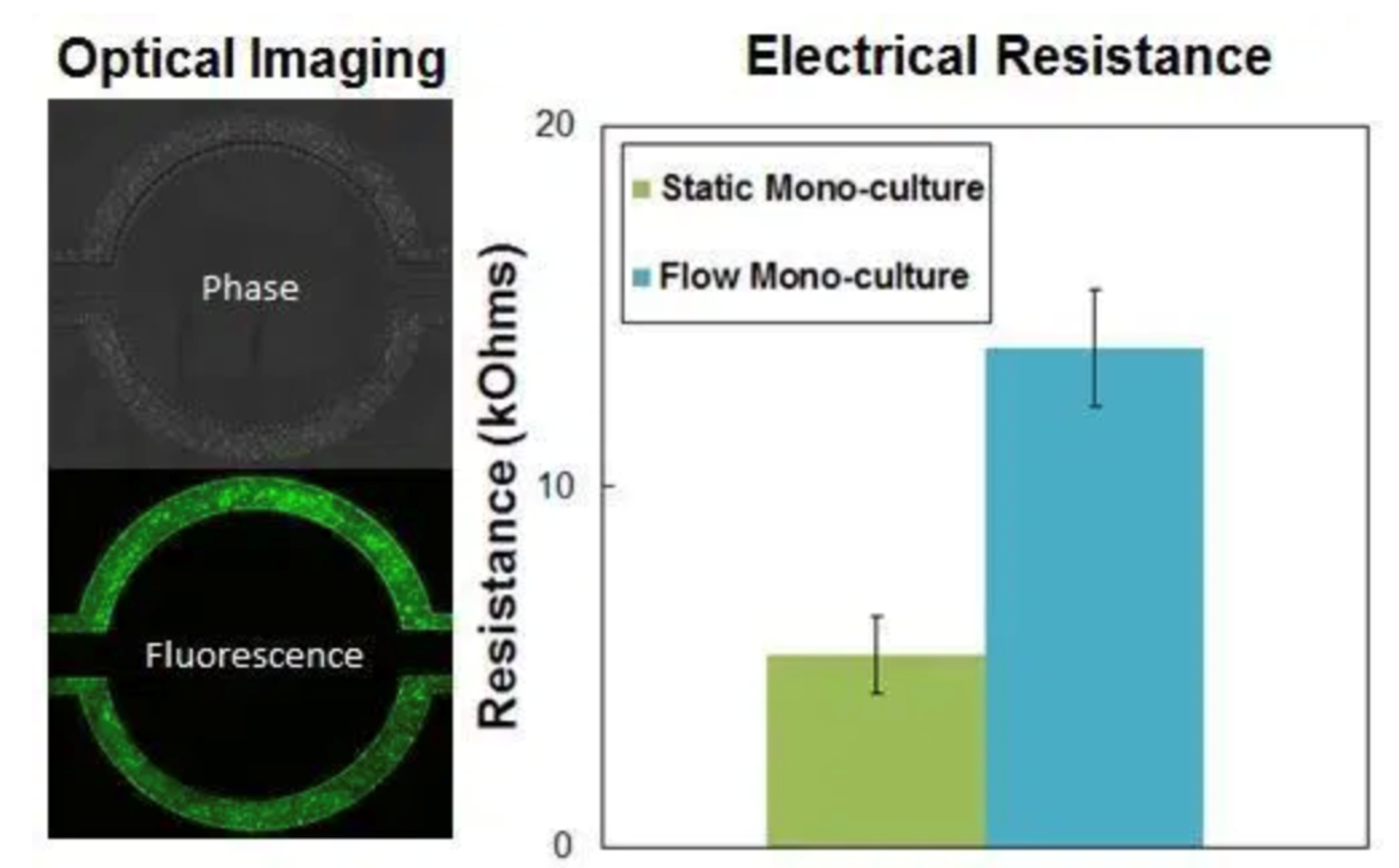
Mono-Culture Model With Brain Endothelial Cells
Shear-induced endothelial cell tight junctions, which cannot be achieved in the Transwell® model, are easily achieved in the SynBBB assay using fluid perfusion. The formation of tight changes can be measured using biochemical or electrical analysis (assessing changes in electrical resistance) with the SynVivo Cell Impedance Analyzer.
Primary endothelial cells are cultured in the vascular channel under physiological fluid flow. Cells are stained for tight junction markers highlighting the increase under fluid flow compared to static conditions. The Cell Impedance Analyzer system is used to measure increases in Ohmic resistance (TEER) associated with the formation of tight junctions.
Real-Time Permeability Assays
Unlike BBB models, which are arranged in top to bottom architecture (i.e., Transwell), small molecule transport can be assessed and quantified in real-time across the SynBBB system due to its side-by-side architecture. A fluorescently labeled drug molecule of interest is perfused through the vascular channels at physiological flow rate. Real-time videos are acquired and analyzed to calculate the rate of permeability into the tissue chamber. Different rates of permeability are observed across the BBB due to tight junctions of endothelial cells.
CAR-T extravasation across the Blood Tumor Barrier
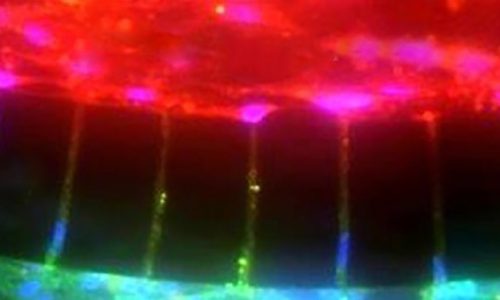
Set-up of blood-brain-tumor barrier (BBTB)-on-CHIP model system using SynBBB. Real-time movie showing immunofluorescence images of the SynBBB blood-brain-tumor-barrier setup in the SynBBB chips with iBECs seeded in the middle channel (phase contrast image) and U87vIII-mKate2 cells (red) seeded in the two outer channels. CAR-F263 (green) perfusion can be seen within the middle iBEC channel. The second video shows examples of CAR-T extravasation across the barrier into the tissue chamber.
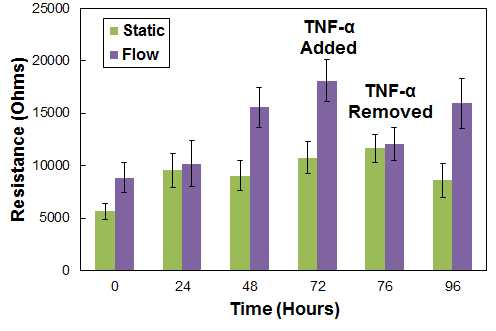
Real-Time Tight Junction Modulation
SynBBB can be used to model inflammation responses. A pro-inflammatory compound, such as TNF-α, is added to mono-cultured endothelial cells to modulate the tight junctions, followed by a period of recovery under perfusion flow. Electrical resistance measurements provide a non-invasive method for real-time monitoring of tight junctions.
Modulation of Inflammation responses in SynBBB model. TNF-alpha induced leakiness in the BBB measured by changes in the resistance across the endothelial cells. Removal of TNF-alpha followed by media perfusion under physiological flow conditions enables recovery of the tight junction leading to increased tight junction formation. Static cells maintain a constant resistance due to lack of tight junctions.
Applications and Endpoints using the SynBBB model:
- Drug Permeability Assays: Evaluate real-time permeability across the BBB.
- Neurotoxicity: Analyze the toxicity effects of chemical, biological, and physical agents on the cells of the BBB.
- Neuroprotection: Evaluate drugs that have a protective effect on the BBB.
- NeuroOncology: Investigate the effects of tumor cells on the BBB.
- NeuroInflammation: Evaluate inflammatory effects on the BBB.
- Biomarker Analysis: Visualize and quantify tight junction or transporter proteins.
- Cell Migration: Visualize and quantify the migration of immune cells.
- Omic Changes: Genomic, proteomic, and metabolic analysis of normal and dysfunctional BBB.
Sample Endpoints
Permeability measurements using fluorescent-tagged molecule or if untagged use mass spec/other readouts from collected effluents, TEER resistance measurements, viability, ROS, real-time imaging of cellular changes, Biomarker analysis, Collect treated cells or effluents for downstream analysis.
Contact us for a Services quote!
Publications Highlighting This Model:
NX210c drug candidate peptide strengthens mouse and human blood-brain barriers
Authors: Chris Greene, Nicolas Rebergue, Gwen Fewell, Damir Janigro, Yann Godfrin, Matthew Campbell and Sighild Lemarchant2* (2024) Fluids and Barriers of the CNS, 21, Article number: 76
Authors: Jez Huang, Ying Betty Li, Claudie Charlebois, Tina Nguyen, Ziying Liu, Darin Bloemberg, Ahmed Zafer, Ewa Baumann, Caroline Sodja, Sonia Leclerc, Gwen Fewell, Qing Liu, Balabhaskar Prabhakarpandian, Scott McComb, Danica B. Stanimirovic and Anna Jezierski (2022) Fluids and Barriers of the CNS, Volume 19, Article number: 38.
A Microfluidic Model of Human Brain (uHuB) for Assessment of Blood-Brain Barrier
Author(s): Tyler D. Brown, Maksymillian Nowak, Alexandra V. Bayles, Balabhaskar Prabhakarpandian, Pankaj Karande, Joerg Lahann, Matthew Helgeson, and Samir Mitragotri
Bioengineering and Translational Medicine. 15: 309 (2019; 4:e10126).
Protein kinase C-delta Inhibition Protects Blood-Brain Barrier from Sepsis-Induced Vascular Damage
Author(s): Yuan Tang, Fariborz Soroush, Shuang Sun, Elisabetta Liverani, Jordan C. Langston, Qingliang Yang, Laurie E. Kilpatrick, and Mohammad F. Kiani. J
Neuroinflammation. 15: 309 (2018).
Trastuzumab Distribution in an In-Vivo and In-Vitro Model of Brain Metastases of Breast Cancer
Author(s): Tori B. Terrell-Hall, Mohamed Ismail Nounou, Fatema El-Amrawy, Jessica I.G. Griffith, and Paul R. Lockman
Oncotarget. 2017; 8:83734-83744
Permeability Across a Novel Microfluidic Blood‑Tumor Barrier Model
Author(s): Tori B. Terrell‑Hall, Amanda G. Ammer, Jessica I. G. Griffith, and Paul R. Lockman
Fluids and Barriers of the CNS (2017) 14:3
A Novel Dynamic Neonatal Blood-Brain Barrier on a Chip
Author(s): S. Deosarkar, B. Prabhakarpandian, B. Wang, J.B. Sheffield, B. Krynska, and M. Kiani
PLOS ONE, 2015
SyM-BBB: A Microfluidic Blood-Brain Barrier Model
Author(s): B. Prabhakarpandian, M.-C. Shen, J.B. Nichols, I.R. Mills, M.S.-Wegrzynowicz, M. Aschner, and K. Pant
Lab on a Chip, 2013, 13, 1093-1101
SynBBB 3D Model – Starter Kits
Includes consumables (12 chips, 100ft tubing, 25 slide clamps, 50 blunt tip needles, and 50 1 ml syringes). Starter kits will also include the pneumatic priming device (required for seeding cells) and the cell impedance analyzer (for TEER configuration only).
Note: Does not include other required consumables such as cells, media, and matrix. Laboratory equipment required includes incubators, inverted microscopes, and syringe pumps.
CAT# | Product Name | Price |
|---|---|---|
402002 | ||
402006 | ||
402004 |
SynBBB 3D Model – Chips
Purchase single chips.
Note: Does not include other required consumables such as cells, media, and matrix. Laboratory equipment required includes incubators, inverted microscopes, and syringe pumps.
CAT# | Product Name | Price |
|---|---|---|
102005-SB3 | ||
108011-SB3 | ||
102003-SB3 | ||
102015-SB3 |