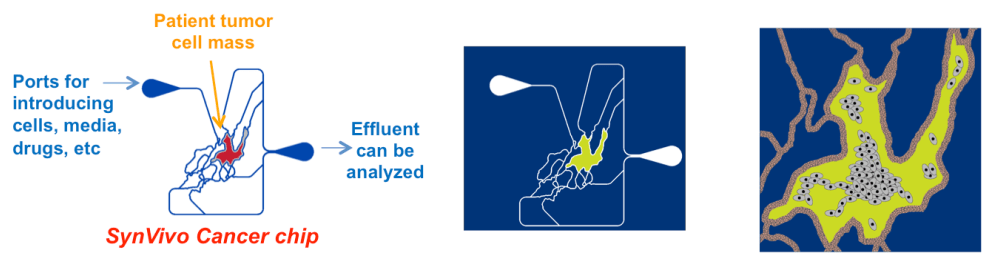
In spite of exciting advances in our understanding of tumor biology that have resulted in a new generation of targeted therapies, multiple challenges continue to impede the identification and delivery of reliably effective cancer medicines. New drugs may provide little therapeutic benefit for some patients, with many approved therapies having lower than 50% response rates. Even with the considerable progress being made with genomic analysis and cancer biomarkers, the current state-of-the-art in cancer therapy, for the most part, requires running experiments in patients to see if the therapy will be effective. Some cancers may progress to an advanced stage while oncologists try to find an effective therapy. As a result many patients are exposed to toxic chemotherapy regimens that don’t halt their cancers. This situation is complicated even further by the routine use of combination therapy to treat many cancers. Clearly, more predictive tools are needed, particularly when a primary cancer treatment fails and the patient needs to undergo an alternative therapy.
A personalized medicine approach can help address the challenge of predicting the therapeutic efficacy of cancer regimens. The best laboratory models currently being used are not reliably predictive because they don’t take into account the personalized nature of the patient’s disease. Most cancer tissue culture and mouse tumor models employ tumor cell lines that do not closely reflect the behavior of the patient’s tumor cells. Each cancer patient is unique because the make-up of each tumor environment is different; and it may evolve as the cancer progresses. Although a patient’s genetic and cancer biomarker profile can aid in optimizing therapy, molecular analysis fails to replicate the actual tumor environment which determines therapeutic efficacy. Having an assay to assess the potential efficacy of these therapies that uses the patient’s own tumor environment, prior to dosing, would be a big step forward.