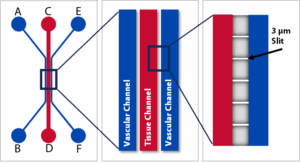
SynBBB Idealized Linear Chip
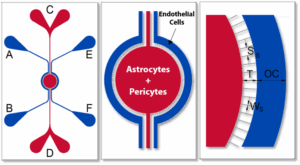
SynBBB Idealized Radial Chip
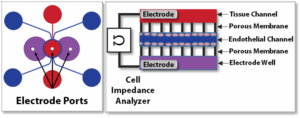
SynBBB Idealized Radial TEER Chip
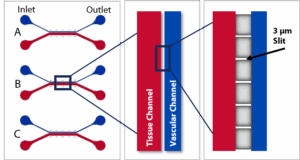
SynBBB 3-PLEX linear Chip
SynBBB™ is SynVivo’s advanced 3D blood-brain-barrier-on-a-Chip model that faithfully replicates in vivo-like neurovascular conditions. By co-culturing brain endothelial cells, astrocytes, and pericytes under controlled flow and shear stress, this blood-brain barrier microfluidics model provides a physiologically relevant platform for CNS drug discovery and translational neuroscience.
With SynBBB™, researchers can:
- Conduct BBB permeability testing for small molecules, biologics, and gene therapies
- Perform BBB transcytosis assays to quantify antibody, peptide, or viral vector transport across the barrier
- Investigate drug-induced and inflammation-driven BBB injury and barrier disruption
- Evaluate therapies that restore function in a leaky blood-brain barrier
- Model neuroinflammation and screen anti-inflammatory therapeutics in a human-relevant system
By integrating dynamic flow and 3D microvascular architecture, SynBBB™ delivers predictive in vitro BBB data that surpasses conventional 2D Transwell assays—accelerating the development of CNS drugs and neurotherapeutics.
Advantages of SynBBB™
- Human-relevant 3D BBB microvascular model
- Dynamic flow and shear stress replicate in vivo conditions
- Real-time imaging & TEER monitoring for mechanistic insights
- Flexible chip designs (linear and radial) for diverse applications
Supports NAMs and non-animal testing strategies aligned with the FDA Modernization Act
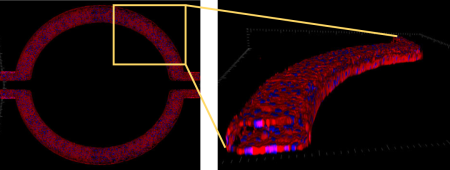
Complete lumen formed with hCMEC/D3 brain endothelial cell line in SynBBB co-culture model
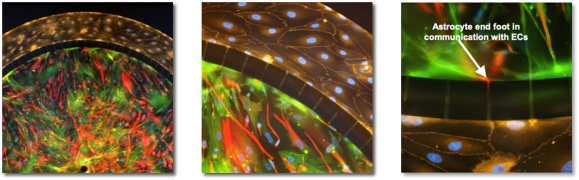
Human BBB-on-a-Chip with primary brain endothelial cells/pericytes/astrocytes
Applications and Assays
Drug Permeability Assays
Neuroprotection
Neuroinflammation
Neurotoxicity
NeuroOncology
Biomarker Analysis
Omic Changes
Publications using SynBBB models
Contract Research Services using SynBBB models
Screen for drug transport, safety, efficacy and more..

SynBBB Models Available
Human BBB-on-a-Chip
- Primary cell based tri-culture BBB model – brain endothelial cells, astrocytes and pericytes
- iPSC-derived tri-culture BBB model with human brain endothelial cells, astrocytes and pericytes
- Cell line based tri-culture BBB model with hCMEC/D3 brain endothelial cell line, primary astrocytes and pericytes
Human BBTB-Barrier-on-a-Chip (Blood-Brain-Tumor-Barrier)
- Brain endothelial cells, astrocytes+glioblastoma
- Brain endothelial cells, tumor cells
Rodent BBB-on-a-chip also available
Sample SynBBB Assays
Drug Transport:
- Real-time permeability of small molecules to biologics
- Receptor-mediated Transcytosis of antibodies and viruses
NeuroInflammation
- Detect drug or disease induced inflammation with immune cell adhesion and migration assays, permeability and TEER
Neuroprotection
- Evaluate drugs that repair the BBB
- Screen with anti-inflammatory therapeutics
Neurotoxicity
- Detect drug or disease induced vascular toxicity
Efficacy
- CAR-T based tumor killing with Blood-Brain-Tumor-Barrier model
Brochures and Technical Manuals
Videos
Purchase SynBBB Products and Instruments
Purchase starter kits, sets of chips, cells/cell lines, instrumentation and training workshops
SynBBB Starter Kits - Select your BBB chip
Important Note: Starter kit does not include required consumables such as cells, media, and matrix. Other required equipment not included is syringe pumps, cell seeding pumps, incubators, and microscopes.
The SynBBB Starter Kit is the fastest way to launch your in vitro BBB assays. Perfect for first-time users, it includes all components needed for setup and operation.
Kit Includes:
- 12 SynBBB Chips (linear or radial configurations)
- Tubing, clamps, needles, and syringes
- Pneumatic priming device for bubble free functionalized models
- Optional TEER configuration with impedance analyzer for barrier integrity monitoring
This complete package allows you to immediately begin CNS drug permeability testing and BBB transcytosis assays in a human-relevant model.
Chip Model | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 402014 | $2,415 | Max: Min: 1 Step: 1 | ||
 | 402002 | $2,415 | Max: Min: 1 Step: 1 | ||
 | 402006 | $2,875 | Max: Min: 1 Step: 1 | ||
 | 402004 | $4,150 | Max: Min: 1 Step: 1 |
SynBBB Chip Options
Choose the chip configuration that best suits your BBB workflow:
SynBBB Linear Chips – Streamlined side-by-side architecture for controlled luminal/abluminal dosing, quantitative permeability studies, and straightforward imaging/analysis
SynBBB Radial Chips – Circular (“brain-slice”) format that supports physiologically relevant flow patterns and expanded assay area for BBB transport and inflammation workflows
Optional TEER Configuration – TEER-enabled setup with impedance analyzer for non-invasive, real-time barrier integrity monitoring during tight junction formation, injury, and recovery
Chip Model | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 102003-SB3 | $1,075 | Max: Min: 1 Step: 1 | ||
 | 108011-SB3 | $425 | Max: Min: 1 Step: 1 | ||
 | 102015-SB3 | $645 | Max: Min: 1 Step: 1 | ||
 | 102005-SB3 | $425 | Max: Min: 1 Step: 1 |
Validated Brain Cells (Primary and Immortalized)
Cells & Cell Lines: SynVivo provides human and animal primary cells and iPSC-derived cell lines validated for use in BBB organ-on-chip assays. Standard options include:
- Human brain microvascular endothelial cells
- Astrocytes and pericytes for co-culture tri-models
- Customizable cell sourcing for specific disease models (e.g., neurodegenerative or rare CNS disorders)
CAT # | Product Name | Price | Qty | |
|---|---|---|---|---|
Syn-10HU-051 | $885 | Max: Min: 1 Step: 1 | ||
Syn-CLU512 | $1,200 | Max: Min: 1 Step: 1 | ||
Syn-10HU-035 | $1,081 | Max: Min: 1 Step: 1 | ||
Syn-10HU-031 | $1,018 | Max: Min: 1 Step: 1 |
Training Workshops
Accelerate adoption of the SynBBB™ platform with scientist-led training:
- 2-Day Workshop – Hands-on introduction to chip seeding, permeability assays, and imaging
- 4-Day Advanced Workshop – Expanded training on BBB transcytosis assays, TEER monitoring, neuroinflammation studies, and mechanistic CNS toxicity testing
Workshops are tailored to your research needs and equip your team with the skills to maximize the platform’s potential.
CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|
SYN48 | SynVivo On-Site Training Workshop – 2 days | $2,750 | Max: Min: 1 Step: 1 | |
SYN96 | SynVivo On-Site Training Workshop – 4 days | $4,500 | Max: Min: 1 Step: 1 |
Syringe Pumps - Instrumentation for Seeding Cells and Maintaining Flow Based Assays
Instrumentation: Ensure seamless operation of your blood-brain barrier microfluidics model with SynVivo’s integrated instrumentation:
- Syringe pumps for controlled cell seeding and drug perfusion under flow
- Stage-top incubators to maintain physiological culture conditions
- Inverted microscopes for real-time visualization of drug transport, immune cell adhesion, and barrier function
- TEER measurement systems for continuous monitoring of barrier integrity
Image | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 301004 | PHD Ultra 6/10 Multi Syringe Rack | $948 | Max: Min: 1 Step: 1 | |
 | 301020 | Multichannel Programmable Syringe Pump – SynVivo NE 1200 | $2,100 | Max: Min: 1 Step: 1 | |
 | 301002 | Pump 11 Elite Nanomite Infusion/Withdrawal Programmable Single Syringe | $3,790 | Max: Min: 1 Step: 1 | |
 | 301003 | PHD Ultra Syringe Pump Programmable 2-Syringe Rack Standard Pressure | $5,651 | Max: Min: 1 Step: 1 |
Tokai Hit Stage Top Incubators
The Tokai Hit incubator offers precision temperature, humidity and CO2 control for long term cell culture on any microscope and optimal conditions for SynVivo’s microfluidic devices.
Image | CAT# | Product Name | Contact Us |
|---|---|---|---|
 | 303015 | ||
 | 303016 | ||
 | 303017 |
















