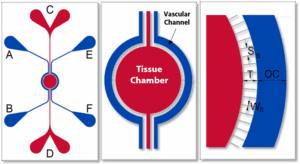
SynRAM Idealized Radial Chip

SynRAM Microvascular Network Chip
SynRAM™ is SynVivo’s advanced inflammation-on-a-chip platform, engineered to replicate human-relevant vascular inflammation under dynamic flow and shear stress. This 3D blood vessel microfluidics model integrates endothelial cells with immune cells (monocytes, neutrophils, macrophages) to provide real-time visualization and quantification of immune cell adhesion, migration, cytokine release, and barrier disruption.
By mimicking the complex vascular microenvironment, SynRAM™ delivers predictive data for immunotoxicology, inflammation-driven disease modeling, and therapeutic screening, surpassing traditional 2D static assays.
Advantages of SynRAM™
- Human-relevant inflammation-on-a-chip model
- Dynamic flow and shear stress for realistic immune cell responses
- Real-time imaging of immune-endothelium interactions
- Multi-cellular co-culture for mechanistic assays
- Supports NAMs & non-animal testing frameworks aligned with the FDA Modernization Act
CRO Services
For turnkey studies, SynVivo offers contract research services (CRO) using the SynRAM™ inflammation model.
- Immunotoxicology Testing – Quantify pro- and anti-inflammatory drug effects
- Immune Cell Adhesion & Migration Assays – Study leukocyte trafficking across vascular endothelium
- Cytokine Release Profiling – Analyze secretion patterns in response to drugs or inflammatory triggers
- Barrier Injury & Recovery Studies – Evaluate endothelial dysfunction and therapeutic repair strategies
- Mechanistic Inflammation Studies – Investigate pathways of cytokine storm, vascular leak, and immune modulation
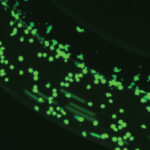
Real-time visualization of rolling, adhesion and migration
Real-time tracking of single-cell migration
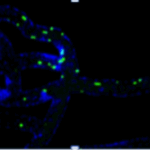
Leukocyte endothelium interactions
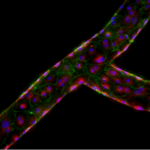
Vessel-on-a-Chip with endothelial cells
Applications and Assays
Inflammation-induced vascular leakage
Inflammation-induced biomarker analysis
Therapeutic drug screening
Screening for activators/inhibitors of inflammation
Publications using SynRAM models
Contract Research Services using SynRAM models
Model Inflammation, immune cell-endothelial interactions, test drug safety
SynRAM Models Available
Monoculture using primary or iPSC derived endothelial cells
- Specific organ matched endothelial cells or HUVECs
Co-Culture with endothelial cells and stromal/tissue cells
- Lung microvascular endothelial cells and epithelial cells
- Brain microvascular endothelial cells and astrocytes/pericytes
Add immune cells to any model
Assays Available
Immune cell rolling, adhesion and migration across the endothelium
Inflammation-induced vascular permeability
Drug-induced vascular injury
Inflammation-induced biomarker analysis, cytokine profiling
Therapeutic drug screening
Screening for cell surface biomarkers
Target identification
Screening for activators/inhibitors of inflammation
Brochures and Technical Manuals
Videos
Purchase SynRAM Products and Instruments
Purchase starter kits, sets of chips, cells/cell lines, instrumentation and training workshops
Starter Kits - Select your chip model
Important Note: Starter kit does not include required consumables such as cells, media, and matrix. Other required equipment not included is syringe pumps, cell seeding pumps, incubators, and microscopes.
SynRAM™ Starter Kit
The SynRAM Starter Kit provides everything needed for first-time use of the inflammation-on-chip system:
Kit Includes:
12 SynRAM Chips (radial or microvascular network formats)
Pneumatic priming device for bubble-free priming and functionalization of microchannels
Tubing, clamps, needles, and syringes
Optional TEER system for monitoring vascular barrier integrity
This all-in-one package enables rapid adoption of in vitro inflammation assays with reproducible results and reliable chip performance.
Chip Model | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
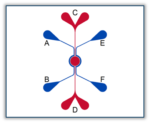 | 401002 | $2,415 | Max: Min: 1 Step: 1 | ||
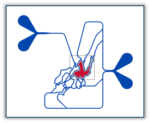 | 401004 | $2,875 | Max: Min: 1 Step: 1 |
SynRAM Model Chips
SynRAM™ Chip Options
Choose the architecture best suited for your inflammation studies:
Radial Chips – Ideal for flow-based immune adhesion and cytokine release assays.
Microvascular Network Chips – Replicate realistic vascular networks for complex immune cell trafficking and extravasation modeling.
Both chip types support high-content imaging and quantitative analysis of inflammatory responses.
Chip Model | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 102008-SR3 | $425 | Max: Min: 1 Step: 1 | ||
 | 105001-SR3 | $550 | Max: Min: 1 Step: 1 |
Human Cells/Cell Lines
Validated cell sources for SynRAM assays include:
Human endothelial cells for vascular lining
Monocytes, neutrophils, macrophages, and PBMCs for immune interaction studies
Customizable iPSC-derived immune cells for translational modeling
.
CAT # | Product Name | Price | Qty | |
|---|---|---|---|---|
Syn-10HU-003-CR10M | $141 | Max: Min: 1 Step: 1 | ||
Syn-10HU-003-CR350M | $894 | Max: Min: 1 Step: 1 | ||
Syn-10HU-003-CR5M | $78 | Max: Min: 1 Step: 1 |
Training Workshops
SynVivo provides hands-on scientist-led workshops to accelerate adoption:
2-Day Training – Chip setup, immune cell assays, cytokine quantification basics
4-Day Training – Advanced immune cell extravasation, mechanistic modeling, and inflammation drug screening
CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|
SYN48 | SynVivo On-Site Training Workshop – 2 days | $2,750 | Max: Min: 1 Step: 1 | |
SYN96 | SynVivo On-Site Training Workshop – 4 days | $4,500 | Max: Min: 1 Step: 1 |
Syringe Pumps - Instrumentation for Seeding Cells and Maintaining Flow Based Assays
SynVivo offers a full suite of compatible organ-on-chip instrumentation to support your toxicology workflows:
- Syringe Pumps – Maintain precise cell seeding and flow conditions
- Stage-Top Incubators – Enable physiologically relevant environments during assays
- Inverted Microscopes – Real-time visualization and quantification of cellular responses
Image | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 301004 | PHD Ultra 6/10 Multi Syringe Rack | $948 | Max: Min: 1 Step: 1 | |
 | 301020 | Multichannel Programmable Syringe Pump – SynVivo NE 1200 | $2,100 | Max: Min: 1 Step: 1 | |
 | 301002 | Pump 11 Elite Nanomite Infusion/Withdrawal Programmable Single Syringe | $3,790 | Max: Min: 1 Step: 1 | |
 | 301003 | PHD Ultra Syringe Pump Programmable 2-Syringe Rack Standard Pressure | $5,651 | Max: Min: 1 Step: 1 |
Tokai Hit Stage Top Incubators
The Tokai Hit incubator offers precision temperature, humidity and CO2 control for long term cell culture on any microscope and optimal conditions for SynVivo’s microfluidic devices.
Image | CAT# | Product Name | Contact Us |
|---|---|---|---|
 | 303015 | ||
 | 303016 | ||
 | 303017 |















