SynTumor Idealized Linear Chip Schematic

SynTumor Idealized Radial Chip Schematic
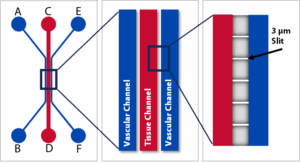
SynTumor Microvascular Network Chip Schematic
SynTumor™ Cancer-on-a-Chip Platform
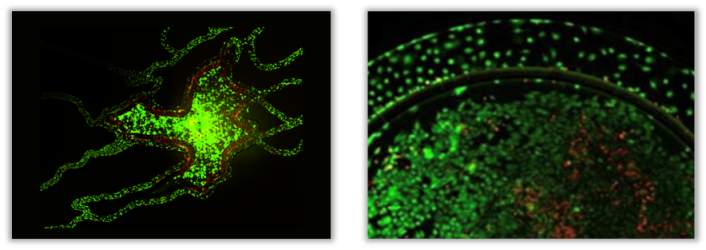
SynTumor™ Cancer-on-a-Chip is SynVivo’s advanced 3D tumor model, designed to replicate in vivo-like tumor microenvironments under dynamic flow conditions. Featuring microvascular networks with controlled pore sizes, this cancer-on-a-chip platform enables quantitative and real-time visualization of cell-cell and cell drug interactions across tumor, stromal, vascular, and immune cells, providing insights into delivery, penetration, and efficacy in a human-relevant in vitro context.
Advantages of SynTumor™
- Human-relevant 3D tumor microvascular modeling
- Controlled flow conditions for physiologically accurate drug delivery studies
- Real-time visualization of tumor-endothelium-drug dynamics
- Modular chip architecture (linear, radial, network) for diverse applications
- Supports NAMs framework for non-animal oncology models
Research Applications
SynTumor™ enables diverse oncology applications with high relevance and precision:
- Tumor Drug Delivery Studies – Investigate how tumor vasculature and flow influence drug distribution and penetration
- Real-Time Mechanistic Insights – Observe interactions between drugs, tumor cells, and the endothelium under dynamic conditions
- Simulated Tumor Microenvironment – Model variable perfusion profiles in tumor microenvironments to investigate the impact on drug efficacy
- Metastasis Modeling – Custom co-culture chips to visualize and quantify tumor cell migration and vascular invasion
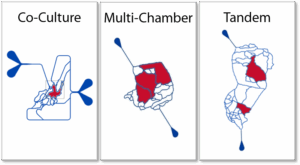
Co-Culture Chips
Microvascular networks recapitulate in vivo scale and vascular geometry. Idealized Network
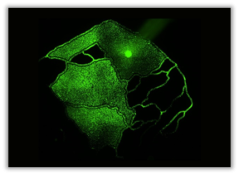
Multi-Chamber Chips
Low or High Perfusion
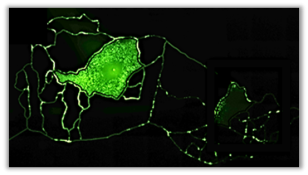
Tandem Co-Culture, Multi-Chamber Chips
Designed with Separate Vascular Network Beds
Applications and Assays
Drug Delivery Screening
Vascular Permeability
Cell-Cell Interactions
Cancer Immunotherapy
Biomarker Analysis
Metastatic Potential Characterization
Publications Using SynTumor Models
Contract Research Services using SynTumor models
Drug delivery under physiological flow, safety, efficacy, metastasis, CAR-T cytotoxicity, multi-organ modeling and more
SynTumor Models Available
Monoculture using tumor cells/cell lines- Human or rodent
Co-Culture with tumor cells and matched endothelial cells
Tri-Culture with tumor, stromal and endothelial cells
- Brain endothelial cells, astrocytes, pericytes and tumor cells and CAR-T tracking for cytotoxicity or neurotoxicity
Assays Available
Efficacy and toxicity screening
Cell proliferation, morphology, and viability
Tumor-induced vascular leakage
Tumor intravasation and extravasation
Tumor immune cell interactions
Drug delivery, uptake, and efficacy
Biomarker analysis
On-chip or off-chip analysis
Brochures and Technical Manuals
Videos
Purchase SynTumor Products and Instruments
Purchase starter kits, sets of chips, instrumentation and training workshops
Starter Kits - Select your chip model
Important Note: Starter kit does not include required consumables such as cells, media, and matrix. Other required equipment not included is syringe pumps, cell seeding pumps, incubators, and microscopes.
SynTumor™ Starter Kit: The Starter Kit offers everything needed to begin your advanced in vitro tumor-on-a-chip modeling workflows:
- 12 Microvascular or Idealized Linear/Radial Chips
- Tubing, clamps, needles, and syringes
- Pneumatic priming device for bubble free functionalized models
- Complete control to model flow-dependent drug delivery under physiologic conditions
Chip Model | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 403002 | $2,415 | Max: Min: 1 Step: 1 | ||
 | 403006 | $2,415 | Max: Min: 1 Step: 1 | ||
 | 403004 | $2,875 | Max: Min: 1 Step: 1 |
SynTumor Model Chips
SynTumor™ Chip Options: Choose the chip architecture that best suits your research needs:
- Microvascular Network Chips – Single or Multi-chamber designs with defined pore sizes (2 µm or 8 µm) for perfusion-based tumor modeling. Variable perfusion profiles for delivery, efficacy and metastasis applications.
- Idealized Radial or Linear Chips – Advanced formats for studying tumor-endothelium interactions and drug transport gradients
Chip Model | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 102004-STu3 | $425 | Max: Min: 1 Step: 1 | ||
 | 102012-STu3 | $425 | Max: Min: 1 Step: 1 | ||
 | 108007-STu3 | $425 | Max: Min: 1 Step: 1 |
Training Workshops
Training Workshops: Enhance your team’s proficiency with expert-led training sessions:
- 2-Day Introduction – Learn chip handling, seeding protocols, and imaging basics
- 4-Day Advanced Training – Dive into dynamic flow modeling, tumor penetration assays, and mechanistic insights
CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|
SYN48 | SynVivo On-Site Training Workshop – 2 days | $2,750 | Max: Min: 1 Step: 1 | |
SYN96 | SynVivo On-Site Training Workshop – 4 days | $4,500 | Max: Min: 1 Step: 1 |
Syringe Pumps - Instrumentation for Seeding Cells and Maintaining Flow Based Assays
Instrumentation: Optimize your workflows with compatible organ-on-chip tools:
- Syringe pumps for controlled perfusion experiments
- Stage-top incubators and inverted microscopes for location-based imaging and real-time quantification
- Perfusion control systems to model variable tumor blood flow and barrier dynamics
Image | CAT# | Product Name | Price | Qty | |
|---|---|---|---|---|---|
 | 301004 | PHD Ultra 6/10 Multi Syringe Rack | $948 | Max: Min: 1 Step: 1 | |
 | 301020 | Multichannel Programmable Syringe Pump – SynVivo NE 1200 | $2,100 | Max: Min: 1 Step: 1 | |
 | 301002 | Pump 11 Elite Nanomite Infusion/Withdrawal Programmable Single Syringe | $3,790 | Max: Min: 1 Step: 1 | |
 | 301003 | PHD Ultra Syringe Pump Programmable 2-Syringe Rack Standard Pressure | $5,651 | Max: Min: 1 Step: 1 |
Tokai Hit Stage Top Incubators
The Tokai Hit stagetop incubator offers precision temperature, humidity and CO2 control for long-term cell culture on any microscope and optimal conditions for SynVivo’s microfluidic devices. Includes a perfusion spacer for flow-based assays.
Image | CAT# | Product Name | Contact Us |
|---|---|---|---|
 | 303015 | ||
 | 303016 | ||
 | 303017 |